If someone would say, a century ago, that there is a shape-memory material, probably no one would believe it. Currently, such material exists. It is not magic; it is science!
NiTiNOL is an intelligent alloy that, after being deformed, can easily go back to its original shape. It has the ability to “remember its shape” depending on temperature. Until the 1980s, it was mainly used for military and aircraft building purposes. However, after the discovery of its biocompatibility it became desirable in medicine. Nowadays, it is far from exclusive material; it is used to produce everyday goods like glasses frames or even earphones.
What does the NiTiNOL acronym mean?
The acronym NiTiNOL come from the composition of the alloy and the place it was invented. Ni and Ti stand for the elements nickel and titanium symbols, respectively. The second part of the acronym—NOL—refers to the Naval Ordnance Laboratory, where the young scientist William J. Buehler discovered it and then developed it in collaboration with Frederick Wang.
This alloy has a near equal proportion of nickel and titanium (49-51% of Ni-Ti). NiTiNOL is a strong, lightweight, and extremely resistant to corrosion material. But, what’s made it so unique is the combination of the superelastic and thermal shape memory properties!
How does NiTiNOL remember its shape?
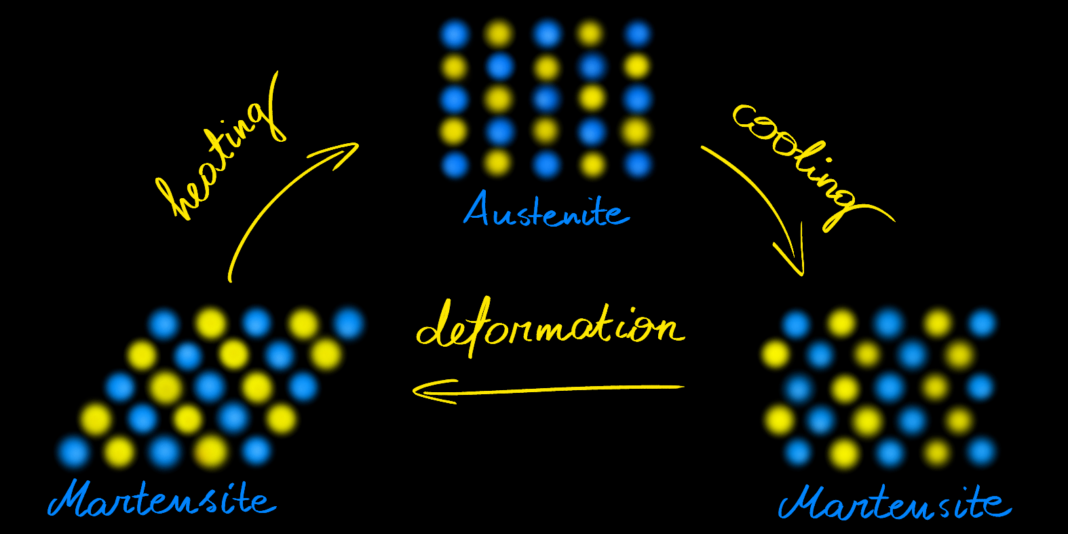
The shape-memory ability of NiTiNOL results from a reversible phase transformation in its crystal structure during temperature change. The alloy exists in two phases: martensite and austenite. Martensite is stable at low temperatures, while austensite is stable at high temperatures. At low temperatures, in the martensite phase, it resembles rubber: it can be easily bent and get a new shape. However, when heated, it enters an austenite phase, becoming strong and stiff, returning to its original shape.
NiTiNOL phase transformation temperature varies from -100°C to 100°C. It can be regulated by changes in its composition and thermo-mechanical treatment. Thanks to that, the temperature of phase transformation can be suited to specific applications. For example, for medical implants, it is equal to human body temperature.
The figure below presents the arrangement of the atoms in NiTiNOL during deformation.
Where is NiTiNOL used?
NiTiNOL is successfully used in robotics. For example, it is applied in artificial muscles (muscle wires) in robotic arms that can lift hundreds or even thousands of times their weight. Since the 1960s, NiTiNOL couplers have been commonly utilized in military F-14 planes as the main component of hydraulic tubing.
Due to its good biological integration, it is widely used in orthopedics. It is suitable for bone plates, internal fixators for long bone shafts, and spinal vertebral spacers, among other applications. However, it stands out in orthodontics. NiTiNOL is the perfect material for connecting the teeth with wires and brackets. In endodontology, it is chosen to fabricate cleaning instruments and prepare the root canals.
Minimally invasive devices such as cardiovascular stents and vena cava filters, where the stent is self-expanding, utilize NiTiNOL. It is also employed in stents in biliary tracts, esophagus, lungs, and ureters.
Do you know that NiTiNOL…?
- It helps for life searching on Mars! — Curiosity’s tires are enjoying the rocky landscapes and low temperature on Mars thanks to shape-memory alloys.

- It revolutionized surgery and rehabilitation — making operations easier than ever before and saving many lives. Specifically, It is used as stents for reinforcement of blood vessels.
NiTiNOL is a remarkably durable material that never forgets. It can withstand crushing better than other alloys and lift objects thousands of times its weight. For its endless variety of applications, it became one of the most essential intelligent materials ever developed.
This article is a joint work of Magdalena Warczak (Institute of Physical Chemistry, Polish Academy of Sciences) and Magdalena Osial (Faculty of Chemistry, University of Warsaw; Institute of Fundamental Technological Research Polish Academy of Sciences). Image Credit – Magdalena Osial
Refernces
- [1] G.B. Kauffman, I. Mayo, “The Story of Nitinol: The Serendipitous Discovery of the Memory Metal and Its Applications,” Chem. Educator, 1997, 2:1–2. DOI:10.1007/s00897970111a.
- [2] M.C. Tanzi, S. Farè, G. Candiani (Authors), “Foundations of Biomaterials Engineering,” Academic Press, 2019.
- [3] J.M. Gallardo Fuentes, P. Gümpel, J. Strittmatter, “Phase Change Behavior of Nitinol Shape Memory Alloys,” Adv. Eng. Mater., 2002, 4:437-452. DOI:10.1002/1527-2648(20020717)4:7<437::AID-ADEM437>3.0.CO;2-8.
- [4] R. Chaudhari, J.J. Vora, D.M. Parikh, “A Review on Applications of Nitinol Shape Memory Alloy” in A.K Parwani, P.L. Ramkumar, K. Abhishek, S.K. Yadav (Editors), “Recent Advances in Mechanical Infrastructure.Proceedings of ICRAM 2020”, Springer Singapore, 2021.
- [5] S.A. Thompson, “An overview of nickel-titanium alloys used in dentistry,” Int. Endod. J., 2000, 33(4):297-310. Doi:10.1046/j.1365-2591.2000.00339.x. PMID: 11307203.
- [6] M. Bahraminasab, B.B. Sahari, “NiTi Shape Memory Alloys, Promising Materials in Orthopedic Applications” in “Shape Memory Alloys – Processing, Characterization and Applications (pp.261-278)”, Publisher: InTech – open science | open minds, 2013. DOI:10.5772/2576.
- [7] B. O’Brien, M. Bruzzi, 1.104 – Shape Memory Alloys for Use in Medicine, Editor(s): P. Ducheyne, Comprehensive Biomaterials, Elsevier, 2011. DOI:10.1016/B978-0-08-055294-1.00014-3.