The beginning of a theory
For more than a century, the Becquerel family worked in the same laboratory in the Jardin des Plantes, later called the Muséum d’Histoire Naturelle, in Paris. In 1878, Henri Becquerel assumed the position of assistant in the Muséum, taking the place left free by his own father.
Antoine Henri Becquerel was the third in a line of four generations of scientists. At 37, he was elected member of the Académie des Sciences (French Academy of Sciences), following the footsteps of his grandfather, Antoine-César, and father, Edmund, who both became presidents of this renowned scientific society.
In 1896, a few copies of Rontgen’s first X-ray images were sent to the Académie. After discussing with Henri Poincaré, Henri Becquerel decided to investigate a possible relation between the emission of this type of radiation and natural luminescence. His initial theory was that minerals made phosphorescent by visible light emit X-rays. Considering that the two previous generations of the Becquerel family had extensively studied the phenomenon of luminescence, it does not come as a surprise that Henri set his mind to research this phenomenon.
The experimental design
The day after his discussion with Poincaré, Becquerel designed an experiment that could confirm his hypothesis. From his father, he had inherited a vast collection of uranium salts, which phosphoresce upon exposure to light.
Two uranium crystals were put over a photographic plate and wrapped in black paper so that light could not reach them. Becquerel’s idea was to expose the plates to sunlight and, in theory, to observe a photographic outline of the crystals. He expected thus to confirm his theory that luminescence and X-rays go hand-in-hand.
The first experiments seemed to confirm his hypothesis. After exposing the photographic plates to solar irradiation, Henri observed the expected outline of the uranium crystals. However, when Becquerel sought validation of his results, things took an unexpected turn.
The accidental twist
Becquerel was doing his experiments in February when one does not expect much sunlight in Paris for several days. Though this may seem like trivial information, it actually determined the new path of Becquerel’s experiment.
It turned out that month was particularly cloudy and grey in the year 1896. Therefore, Henri could not expose the experimental plates to bright sunlight for several days. The photographic plates were, for that reason, kept in a drawer, waiting for the sun to shine again.
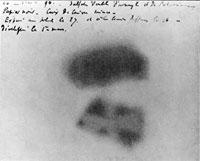
exposed on March 1st, 1896
On the 1st of March, Henri opened the drawer and examined the plates, expecting to see a blunt outline of the uranium crystals. But, to his surprise, he was able to clearly see the image of the uranium crystals. On top of that, he found that the images were as bright as the first pictures that had been exposed to bright sunlight.
It didn’t take Becquerel much time to realize that his initial hypothesis was wrong. Sunlight was unnecessary for the emission of radiation. Thus, another unknown source was responsible for this phenomenon. On March 2nd, 1896, Becquerel reported in the Académie that the uranium salts emitted radiation with no stimulation from sunlight.
After a few months of further experiments, he reported his additional conclusions to the Académie. The most likely explanation was that uranium salts stored energy and non-phosphorescent salts also emit invisible radiation with the same intensity as the phosphorescent ones.
Further work on radioactivity
Though the discovery of radioactivity is attributed to Becquerel, the term was first used by Pierre and Marie Curie. Prompted by Becquerel’s results, Marie Curie investigated whether it was possible to achieve the same results with other minerals. It took the Curie couple four years to discover three new radioactive substances- polonium, thorium, and radium, which were much more active than uranium. They reported that their discoveries were made possible “only due to the new investigation procedure made possible by the Becquerel rays”.
The work on radioactivity was explored in parallel by many others researchers, including Rutherford. Together with Frederick Soddy, Rutherford studied the properties of radioactive decay and explained radioactivity as the spontaneous disintegration of atomic nuclei, which result in the emission of energy in the form of alpha, beta or gamma rays.
In 1903, Becquerel won the Nobel Prize in Physics, “in recognition of the extraordinary services he has rendered by his discovery of spontaneous radioactivity”. The prize was shared with Pierre and Marie Curie, “in recognition of the extraordinary services they have rendered by their joint researches on the radiation phenomena discovered by Professor Henri Becquerel”. Five years later, Rutherford won the Nobel Prize in Chemistry for his work in the disintegration of elements and the chemistry of radioactive substances. In that same year, Antoine Henri Becquerel died of a heart attack, at the age of 55.
The discovery of radioactivity was surrounded by a series of accidents and unplanned events, starting with the discovery of the X-rays by Roentgen. But attributing the credits of such an important discovery solely to chance or fortune does no justice to the hard work of Becquerel.
References:
Allisy, A. (1996). Henri Becquerel: The Discovery of Radioactivity Radiation Protection Dosimetry, 68 (1), 3-10 DOI: 10.1093/oxfordjournals.rpd.a031848
Nobelprize.org – Henri Becquerel
Wired – March 1, All Aglow
APS Physics – March 1, 1896: Henri Becquerel Discovers Radioactivity





